
分类: Independent Work Prior to DHU
年份: 2021 2020 2019 2018 2017 2015 2014 2013 2012 2010 2009
Independent Work
2021
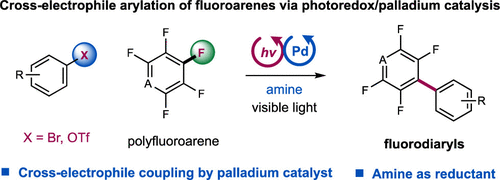
32. “Selective Fluoromethyl Couplings of Alkynes via Nickel Catalysis”
Li, H.; Wang, F.; Zhu, S.; Chu, L.* Angew. Chem. Int. Ed. 2021, DOI: 10.1002/anie.202116725

31. “Selective Ni-catalyzed cross-electrophile coupling of alkynes, fluoroalkyl halides, and vinyl halides”
Dai, Y.; Wang, F.; Zhu, S.; Chu, L.* Chin. Chem. Lett. DOI: 10.1016/j.cclet.2021.12.050.
# Special issue for Fluorine Chemistry

29. “Recent Advances in Photoredox/Nickel Dual-Catalyzed Difunctionalization of Alkenes and Alkynes”
Xu, L.; Wang, F.; Chen, F.; Zhu, S.; Chu, L.* Chin. J. Org. Chem. 2021, 41, DOI: 10.6023/cjoc202109002.
# Invited review
27. “Ni-Catalyzed Ligand-Controlled Regiodivergent Reductive Dicarbofunctionalization of Alkenes”
Li, X.; Chu, L.* Chin. J. Org. Chem. 2021, 41, 4101-4102. DOI: 10.6023/cjoc202100076.
# Invited Highlight
24. “Photoinduced triiodide-mediated [3+2] cycloaddition of N-tosyl aziridines and alkenes”
Li, Y,-B.#; Chen, F.: Zhu, S,-Q.; Chu, L.* Org. Chem. Front., 2021, 8, 2196-2202.
![Graphical abstract:Photoinduced triiodide-mediated [3 + 2] cycloaddition of N-tosyl aziridines and alkenes](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1QO00102G&imageInfo.ImageIdentifier.Year=2021)
Qin, J.; Zhu, S,-Q.; Chu, L.* Organometallics., 2021, 40, 2246-2252.
Feng, X.-L.; Guo, L.; Zhu, S.-Q.; Chu, L.* Synlett., 2021, 32, 1519-1524.

21. “Silver‐Enabled General Radical Difluoromethylation Reaction with TMSCF2H”
Yang, J.#; Zhu, S,-Q.; Wang, F.; Qing, F.-L.; Chu, L.* Angew. Chem. Int. Ed., 2021, 60, 4300-4306.

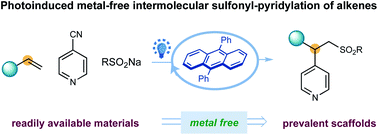
20. “Organic-photoredox-catalyzed three-component sulfonylative pyridylation of styrenes”
Wang, F.; Qin, J.; Zhu, S.; Chu, L.* RSC Adv. 2021, 11, 142-146.
2020
19. “Cu-Catalyzed Regio-and Stereo-selective 1,3-and 1,4-Diborylations of CF3-Containing 1,3-Enynes”
Zhu, S.; Chu, L.* Chin. J. Org. Chem. 2020, 40, 3980-3981.
# Invited Highlight
Guo, L.#; Yuan, M.-B.#; Zhang, Y.-Y.; Wang, F.; Zhu, S.-Q.; Osvaldo Gutierrez*; Chu, L.*
J. Am. Chem. Soc., 2020, 142, 20390.

17. “Enantioselective Three-Component Fluoroalkylarylation of UnactivatedOlefins Through Nickel-Catalyzed Cross-Electrophile Coupling”
Tu, H.-Y.#; Wang, F.#; Huo, L.-P.; Li, Y.-B.; Zhu, S.-Q.; Zhao, X.; Li, H.; Qing, F.-L.; Chu, L.*
J. Am. Chem. Soc., 2020,142, 9604.

16. “Sequential C-O Decarboxylative Vinylation/C-H Arylation of Cyclic Oxalates via Nickel-Catalyzed Multicomponent Radical Cascade”
Li, H.#; Guo, L.#; Feng, X.-L.; Huo, L.-P.; Zhu, S.-Q.; Chu, L.* Chem. Sci., 2020,11, 4904.
15. “Recent advances in photoredox and nickel dual-catalyzed cascade reactions: pushing theboundaries of complexity”
Zhu, C.; Yue, H.-F.; Chu, L.; Rueping, M. Chem. Sci., 2020,11, 4051-4064.
14.“Recent Advances in Nickel-Catalyzed Three-Component Difunctionalization of Unactivated Alkenes”
Tu, H.-Y.; Zhu, S.-Q.*;Qing, F.-L.; Chu, L.*, Synthesis., 2020,52, 1346-1356.
2019
Song, F.; Wang, F.; Guo, L.; Feng, X.-L.; Zhang, Y.-Y.; Chu, L.*, Angew.Chem.Int.Ed., 2019,59, 177-181.

12. “Bisphosphonium salt: an effective photocatalyst for the intramolecular hydroalkoxylation of olefins”
Cheng, H.; Wang, X.; Chang, L.; Chen, Y.-L.; Chu, L.*; Zuo, Z.-W.*, Sci. Bull., 2019,64, 1896-1901.
11. 'Selective, Intermolecular Alkylarylation of Alkenes via Photoredox/Nickel Dual Catalysis”
Guo, L.#; Tu, H.-Y.#; Zhu, S.-Q.; Chu, L.*, Org. Lett., 2019, 21, 4771-4776.

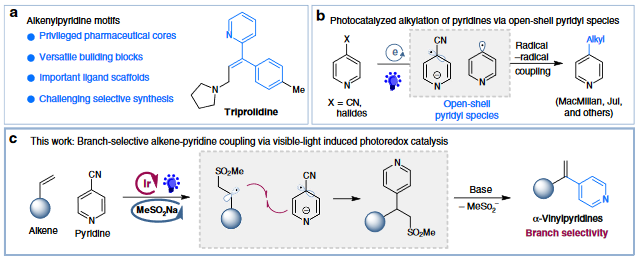
10. “Photoredox-catalyzed branch-selective pyridylation of alkenes for the expedient synthesis of Triprolidine”
Zhu, S.-Q.; Qin, J.; Wang, F.; Li, H.; Chu, L.*, Nat. Commun., 2019,10, 749
9. “Intermolecular, redox-neutral azidoarylation of alkenes via photoredox catalysis”
Chen, J.#; Zhu, S.-Q.#; Qin, J.; Chu, L.*, Chem. Commun., 2019,55, 2336-2339.

2018
8. “Solvent-tuned chemoselective carboazidation and diazidation of alkenes via iron catalysis”
Xu, L.; Chen, J.; Chu, L.*, Org. Chem. Front.,2018, 6, 512-516.
Tu, H.-Y.; Zhu, S.-Q.; Qing, F.-L.; Chu, L.*, Chem. Commun. 2018, 54, 12710-12713.
Chen, D.; Xu, L.; Long, T.; Zhu, S.-Q.; Yang, J.; Chu, L.*, Chem. Sci., 2018, 9, 9012-9017.
Guo, L.; Song, F.; Zhu, S.-Q.; Li, H.; Chu, L.*, Nat. Commun., 2018, 9, 4543.

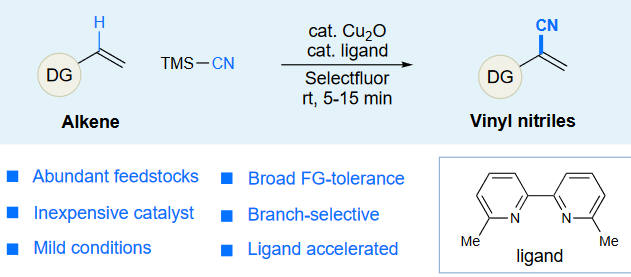
4. “Ligand-Accelerated, Branch-Selective Oxidative Cyanation of Alkenes”
Yang, J.; Li, H.; Qin, J.; Song, F.; Zhang, J.; Qing, F.-L.; Chu, L.*, Sci. Bull., 2018, 63, 1479.
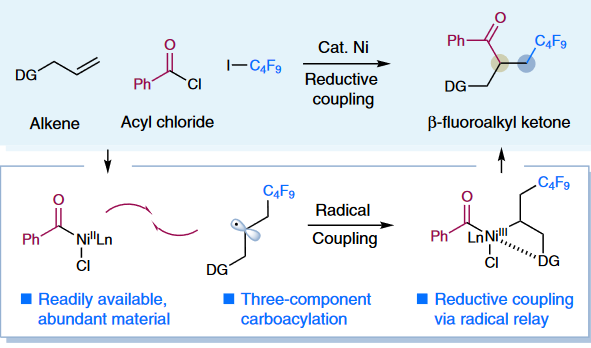
3. “Intermolecular selective carboacylation of alkenes via nickel-catalyzed reductive radical relay”
Zhao, X.#; Tu, H.-Y.#; Guo, L.; Zhu, S.-Q.; Qing F.-L.; Chu, L.*, Nat. Commun., 2018, 9, 3488.
X-MOL 评论:http://www.x-mol.com/news/14833
东华大学新闻链接:https://mp.weixin.qq.com/s/q6dtfPQiblX9HCqSwbClSA
2. “Catalytic, Metal-free Sulfonylcyanation of Alkenes via Visible light Organophotoredox Catalysis”
Sun, J.#; Li, P.#; Guo, L.; Yu, F.; He, Y.-P.*; Chu, L.*, Chem. Commun., 2018, 54, 3162-3165.

2017
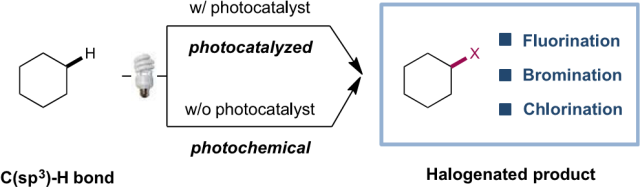
1. “Visible-light-induced Halogenation of Aliphatic C-H Bonds”,
Tu, H.; Zhu, S.; Qing, F.-L.; Chu, L*,
Tetrahedron Lett. 2017, 59, 173-179. (Invited Review)
Prior to DHU
2015
Liu, J.-B.; Chen, C.; Chu, L. Chen, Z.-H.; Xu, X.-H.; Qing, F.-L.*,
Angew. Chem. Int. Ed. 2015, 54, 11839-11842.
Chu, L.; Lipshultz, J. M.; MacMillan, D. W. C.*,
Angew. Chem. Int. Ed. 2015, 54, 7929−7933 (Very Important Paper).
2014
Chu, L.; Ohta, C.; Zuo, Z.; MacMillan, D. W. C.*,
J. Am. Chem. Soc. 2014, 136, 10886−10889.
21. “Merging Photoredox with Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides”
Zuo, Z.; Ahneman, D.; Chu, L.; Terrett, J.; Doyle, A. G.*; MacMillan, D.W. C.*,
Science 2014, 345, 437−440.
Chu, L.; Qing, F.-L.*,
Acc. Chem. Res. 2014, 47, 1513−1522.
2013
Lin, Q.; Chu, L.; Qing, F.-L.*,
Chin. J. Chem. 2013, 31, 885-891.
Jiang, X.; Chu, L.; Qing, F.-L.*,
New J. Chem. 2013, 37, 1736−1741.
Chen, J.-L.; Chu, L.; Qing, F.-L.*,
J. Fluorine Chem. 2013, 152, 70−76.
16. “Electrophilic Trifluoromethylthiolation of Allylsilanes with Trifluoromethanesulfanamide”
Liu, J.; Chu, L.; Qing, F.-L.*,
Org. Lett. 2013, 15, 894−897.
15. “Silver-Catalyzed Hydrotrifluoromethylation of Unactivated Alkenes with CF3SiMe3”
Wu, X.; Chu, L.; Qing, F.-L.*,
Angew. Chem. Int. Ed., 2013, 52, 2198−2202.
Wu, X.; Chu, L.; Qing, F.-L.*,
Tetrahedron Lett. 2013, 54, 249−251.
2012
Jiang, X.-Y.; Chu, L.; Wang, R.-W.; Qing, F.-L.*,
Tetrahedron Lett. 2012, 53, 6853−6857.
Chen, C.; Chu, L.; Qing, F.-L.*,
J. Am. Chem. Soc. 2012, 134, 12454−12457.
Jiang, X.; Chu, L.; Qing, F.-L.*,
Org. Lett.2012, 14, 2870−2873.
Chu, L.; Qing, F.-L.*,
Org. Lett. 2012, 14, 2106−2109.
Chu, L.; Qing, F.-L.*,
Synthesis2012, 44, 1521−1525.
Jiang, X.; Chu, L.; Qing, F.-L.*,
J. Org. Chem.2012, 77, 1251−1257.
Chen, C.; Xie, Y. Chu, L.; Wang, R. Zhang, X. Qing, F.-L.*,
Angew. Chem. Int. Ed., 2012, 51, 2492−2495.
6. “Copper-Catalyzed Direct C-H Oxidative Trifluoromethylation of Heteroarenes”
Chu, L.; Qing, F.-L.*,
J. Am. Chem. Soc., 2012, 134, 1298−1304.
2010
5. “Copper-Mediated Oxidative Trifluoromethylation of Boronic Acids”
Chu, L.; Qing, F.-L.*,
Org. Lett., 2010, 12, 5060–5063.
4. “Copper-Mediated Aerobic Oxidative Trifluoromethylation of Terminal Alkynes with Me3SiCF3”
Chu, L.; Qing, F.-L.*,
J. Am. Chem. Soc., 2010, 132, 7262−7263.
Chu, L.; Qing, F.-L.*,
Chem. Commun., 2010, 46, 6285−6287.
2. “Cu(II)-Mediated Methylthiolation of Aryl C−H Bonds with DMSO”
Chu, L.; Yue, X.; Qing, F.-L.*,
Org. Lett., 2010, 12, 1644−1647.
2009
1. “CuBr-Catalyzed Oxidative Difluoromethylation of Tertiary Amines with Difluoroenol Silyl Ethers”
Chu, L.; Zhang, X.; Qing, F.-L.*,
Org. Lett., 2009, 11, 2197−2200.